Spotlight On: Oncology


This month we cast a spotlight on articles, SurgeryU videos, and Journal of Minimally Invasive Gynecology (JMIG) article recommendations from the AAGL Oncology Special Interest Group (SIG) led by Chair, Dario R. Roque, MD.
Access to SurgeryU and JMIG are two of the many benefits included in AAGL membership. The SurgeryU library features high-definition surgical videos by experts from around the world. JMIG presents cutting-edge, peer-reviewed research, clinical opinions, and case report articles by the brightest minds in gynecologic surgery.
SurgeryU video recommendations by our SIGs are available for public access for a limited time. The links to JMIG article recommendations are accessible by AAGL members only. For full access to SurgeryU, JMIG, CME programming, and member-only discounts on meetings, join AAGL today!
SIG Recommended SurgeryU Video #1:
Fertility-Sparing Cornual Wedge Resection for Management of GTN
Ryan S. Perry, MD, David A. Iglesias, MD, Jessica N. Sosa-Stanley, MD, James N. Casey, MD
This video features a case that illustrates the concepts of fertility-sparing surgery for gynecologic cancers, which is the focus of the Oncology SIG’s postgraduate course at this year’s AAGL Meeting.
SIG Recommended SurgeryU Video #2:
Multiquadrant Robotic Assisted Primary Cytoreduction for Stage IIIC Ovarian Cancer
Peter C. Lim, MD, Lauren L. Kennedy, BS
The LANCE Trial is evaluating the role of MIS in ovarian cancer interval debulking surgery. Preliminary findings from the lead-in pilot phase of the LANCE Trial demonstrated comparable complete gross resection rates between the MIS and laparotomy arms. Furthermore, the MIS arm showed encouraging perioperative benefits, such as shorter median postoperative hospital stays, lower estimated blood loss, and fewer postoperative complications. This video highlights the feasibility of minimally invasive surgery for the management of advanced ovarian cancer.
JMIG Article Recommendation #1:
Use of the Frailty “Timed Up and Go” Test to Predict Perioperative Complications in Patients Undergoing Gynecologic Cancer Surgery
Mary Katherine Anastasio, MD, Allison Schwalb, BS, Katherine Penvose, BA, Donna Niedzwiecki, PhD, Gloria Broadwater, MS, Leah McNally, MD
This study assesses the utility of the Timed Up and Go (TUG) test as a frailty screening tool in patients undergoing gynecologic oncology surgery. The authors found that slower TUG times correlated with older age, malnutrition, and comorbidities Frail patients identified by TUG are more likely to require perioperative blood transfusions and experience postoperative complications, although the test does not reliably predict length of hospital stay or discharge disposition. The predictive value of TUG is more limited in minimally invasive laparoscopic procedures. Overall, the TUG test is a simple, objective, and practical measure of functional status that can aid in preoperative risk stratification and guide targeted interventions.
JMIG Article Recommendation #2:
Trends in Volume of Benign Hysterectomies Performed by Gynecologic Oncologists
Annalyn M. Welp, MD, Linda R. Duska, MD, Laura N. Homewood, MD
The investigators queried the National Surgical Quality Improvement Program (NSQIP) and identified an increasing volume of benign hysterectomies being performed. Gynecologic oncologists are performing a growing proportion of these benign cases, including hysterectomies in patients with a history of prior abdominal surgery and uterine weights >500g. These findings carry substantial implications for resident education, fellowship training, and inter-specialty referral pathways.
SurgeryU Video and JMIG Article Recommendations By:
Dario R. Roque, MD

Dr. Roque is Chair of the AAGL Oncology SIG, Deputy Editor on the JMIG Editorial Board, and Associate Professor of Gynecologic Oncology in the Department of Obstetrics & Gynecology at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
Robotic Hyperthermic Intraperitoneal Chemotherapy: Minimally Invasive Techniques for Modern Oncologic Therapeutics
Hyperthermic Intraperitoneal Chemotherapy (HIPEC) has been described in the medical literature for gynecologic cancer since the 1990’s. Within the past decade, multiple studies have shed light on the efficacy of HIPEC, especially in epithelial ovarian cancer (EOC) [1-3].
A 50-year-old woman had undergone an appendectomy for acute appendicitis. Pathology subsequently returned as mucinous adenocarcinoma, invading the muscularis propria, and with evidence of perforation. Following the appropriate staging procedure which included a right colectomy, the final stage was pT2N0. She continued with routine surveillance including interval computerized tomography (CT) scans as well as colonoscopy. 36 months after her staging procedure, the patient had a Positron Emission Topography (PET) scan that revealed FDG avidity of a 2cm lesion consistent with peritoneal carcinomatosis/metastatic disease. This recurrence was treated with six cycles of combination folinic acid, fluorouracil, and oxaliplatin (FOLFOX) with bevacizumab. The most recent CT imaging was consistent with stable disease (1.9cm lesion). After appropriate counseling and with shared medical decision making, and in conjunction with a referral to gynecologic oncology, the patient was consented for robotic-assisted cytoreductive surgery, bilateral salpingo-oophorectomy, and intraoperative HIPEC.
Two small vertical incisions were carried down to the fascia, and a mini gelport was fashioned in both respective locations, one which was subxiphoid, and one at the prior cesarean section scar site [Figure 1-2].
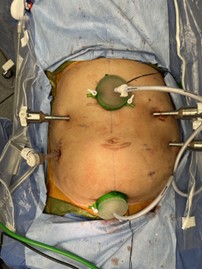 Figure 1: HIPEC Port Sites from a Vertical Vantage Point
Figure 1: HIPEC Port Sites from a Vertical Vantage Point
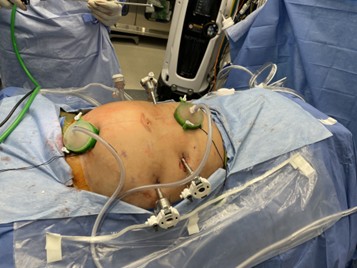 Figure 2: HIPEC Port Sites from the Surgeon’s Perspective
Figure 2: HIPEC Port Sites from the Surgeon’s Perspective
The catheter tubing for intraperitoneal chemotherapy was positioned such that the inflow was into the pelvis, with the outflow catheter above the liver. Perfusate saline was introduced and once approaching 42 degrees, 40mg of Mitomycin-C was introduced and the abdomen and pelvis gently rocked for 90 minutes. Chemotherapy was then drained, and the patient’s peritoneal cavity was irrigated with 6 liters of sterile water.
Two primary studies have shown statistically significant efficacy for treatment of malignancy with HIPEC. One study of 245 patients in stage III EOC demonstrated recurrence free survival advantage (14 vs 11 months) as well as overall survival benefit (45 vs 33 months) [4]. Another study of 184 patients with stage III/IV ovarian cancer revealed that the subgroup receiving HIPEC that followed neoadjuvant chemotherapy (NACT) and interval cytoreductive surgery were found to have similar progression free survival and increased median overall survival (62 vs 48 months) [5]. Evidence thus supports that women with advanced ovarian cancer who receive NACT may benefit from HIPEC during cytoreductive surgery in facilities that provide available expertise, and careful patient selection who undergo R0 resection.
National consensus has proposed HIPEC with single agent cisplatin and the OVHIPEC-1 regimen [6]. While no specific methodology has been established, and medical literature investigating open versus closed approached prove no superiority in one technique versus another, this case highlights an efficient minimally invasive approach in a comfortable and reproducible operative set up for the minimally invasive oncologic surgeon [6].
References:
- van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan 18;378(3):230-240. doi: 10.1056/NEJMoa1708618. PMID: 29342393.
- Zivanovic O, Chi DS, Zhou Q, Iasonos A, Konner JA, Makker V, Grisham RN, Brown AK, Nerenstone S, Diaz JP, Schroeder ED, Langstraat CL, Paroder V, Lakhman Y, Soldan K, Su K, Gardner GJ, Andikyan V, Guo J, Jewell EL, Long Roche K, Troso-Sandoval T, Lichtman SM, Moukarzel LA, Dessources K, Abu-Rustum NR, Aghajanian C, Tew WP, Beumer J, Sonoda Y, O’Cearbhaill RE. Secondary Cytoreduction and Carboplatin Hyperthermic Intraperitoneal Chemotherapy for Platinum-Sensitive Recurrent Ovarian Cancer: An MSK Team Ovary Phase II Study. J Clin Oncol. 2021 Aug 10;39(23):2594-2604. doi: 10.1200/JCO.21.00605. Epub 2021 May 21. PMID: 34019431; PMCID: PMC8330970.
- Walker JL, Brady MF, Wenzel L, Fleming GF, Huang HQ, DiSilvestro PA, Fujiwara K, Alberts DS, Zheng W, Tewari KS, Cohn DE, Powell MA, Van Le L, Davidson SA, Gray HJ, Rose PG, Aghajanian C, Myers T, Alvarez Secord A, Rubin SC, Mannel RS. Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2019 Jun 1;37(16):1380-1390. doi: 10.1200/JCO.18.01568. Epub 2019 Apr 19. Erratum in: J Clin Oncol. 2019 Sep 1;37(25):2299. doi: 10.1200/JCO.19.01365. PMID: 31002578; PMCID: PMC6544459.
- Aronson SL, Lopez-Yurda M, Koole SN, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van Gent MDJM, Arts HJG, van Ham MAPC, van Dam PA, Vuylsteke P, Aalbers AGJ, Verwaal VJ, Van de Vijver KK, Aaronson NK, Sonke GS, van Driel WJ. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer (OVHIPEC-1): final survival analysis of a randomised, controlled, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1109-1118. doi: 10.1016/S1470-2045(23)00396-0. Epub 2023 Sep 11. PMID: 37708912.
- Lim MC, Chang SJ, Park B, Yoo HJ, Yoo CW, Nam BH, Park SY; HIPEC for Ovarian Cancer Collaborators. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022 May 1;157(5):374-383. doi: 10.1001/jamasurg.2022.0143. PMID: 35262624; PMCID: PMC8908225.
- Bhatt A, Glehen O, Zivanovic O, Brennan D, Nadeau C, Van Driel W, Bakrin N. The 2022 PSOGI International Consensus on HIPEC Regimens for Peritoneal Malignancies: Epithelial Ovarian Cancer. Ann Surg Oncol. 2023 Dec;30(13):8115-8137. doi: 10.1245/s10434-023-13932-3. Epub 2023 Aug 10. PMID: 37561343.
About the Author:
Michael Kee-Ming Shu, MD
Jessie Hollingsworth, MD
Lauren Gottshall, MD
Haejin In, MD
Ruth Stephenson, DO
 Dr. Shu is a board member of the AAGL Oncology SIG and immediate former MIGS Fellowship faculty at Henry Ford Health System in Detroit, Michigan. Dr. Shu is currently a gynecologic oncology fellow at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
Dr. Shu is a board member of the AAGL Oncology SIG and immediate former MIGS Fellowship faculty at Henry Ford Health System in Detroit, Michigan. Dr. Shu is currently a gynecologic oncology fellow at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
 Dr. Hollingsworth is a Gynecologic Oncology Fellow in the Department of Gynecologic Oncology at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
Dr. Hollingsworth is a Gynecologic Oncology Fellow in the Department of Gynecologic Oncology at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
 Dr. Gottshall is a Gynecologic Oncology Fellow in the Department of Gynecologic Oncology at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
Dr. Gottshall is a Gynecologic Oncology Fellow in the Department of Gynecologic Oncology at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
 Dr. In is a Surgical Oncologist in the Department of Surgical Oncology at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
Dr. In is a Surgical Oncologist in the Department of Surgical Oncology at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
 Dr. Stephenson is a Gynecologic Oncologist in the Department of Gynecology Oncology at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.
Dr. Stephenson is a Gynecologic Oncologist in the Department of Gynecology Oncology at Rutgers Cancer Institute of New Jersey in New Brunswick, New Jersey.

MIS in Ovarian Cancer: Current Benefits, Future Directions, and the LANCE Perspective
Minimally invasive surgery (MIS) has progressively carved out a significant role in the surgical management of ovarian cancer (OC). While laparotomy remains the standard surgical intervention for the majority of OC cases, MIS offers a compelling alternative in specific cases, such as in patients with early-stage disease and in carefully selected patients with oligometastatic recurrences. The rationale for integrating MIS into ovarian cancer treatment stems from its potential to decrease surgical morbidity and accelerate patient recovery. Given that a substantial majority of OC patients are diagnosed at advanced stages, any approach that can lessen the physical burden of surgery while maintaining oncological efficacy is highly desirable. However, the journey towards its universal acceptance, especially for advanced OC, is contingent upon the accumulation of robust evidence from large-scale randomized controlled trials.
In the context of early-stage ovarian cancer, where the prognosis is generally favorable with a 5-year relative survival rate exceeding 90%, MIS has gained considerable acceptance. Studies have consistently demonstrated that MIS can achieve survival outcomes comparable to those seen with traditional open approaches. Patients undergoing minimally invasive surgery for early-stage OC can also benefit from the well-established benefits of MIS, including reduced blood loss, lower rates of postoperative complications, and significantly less postoperative pain. All of which translates into a faster recovery and shorter hospital stays.
The use of MIS in Interval Debulking Surgery (IDS) following neoadjuvant chemotherapy (NACT) remains controversial. Several small studies have demonstrated that MIS is feasible for IDS and that survival outcomes are comparable to those of traditional laparotomy. In addition, patients undergoing MIS for IDS typically experience reduced perioperative complications, including lower blood loss and shorter hospital stays. A larger study utilizing data from the US National Cancer Database and propensity score matching, compared minimally invasive surgery (MIS) to open laparotomy for interval debulking in advanced ovarian cancer patients following neoadjuvant chemotherapy. MIS was associated with improved overall survival (or at least non-inferiority), lower 30-day and 90-day postoperative mortality rates, shorter hospital stays, and lower residual disease (1). However, the investigators acknowledged limitations such as a lack of detailed clinical data and emphasized the need for further prospective research to define optimal candidates and confirm long-term outcomes.
While observational studies and retrospective analyses have demonstrated encouraging results for the role of MIS in ovarian cancer IDS, the absence of large-scale randomized controlled trials (RCTs) has historically limited the ability to draw definitive conclusions. The LANCE trial, an international, prospective, randomized, multicenter, non-inferiority phase III trial, with a primary endpoint of disease-free survival, is specifically designed to address this important question. Preliminary findings from the lead-in pilot phase of the LANCE study have already demonstrated the feasibility of conducting such a comprehensive RCT. The trial successfully achieved an adequate patient accrual rate, maintained a low rate of treatment crossover, and demonstrated comparable complete gross resection rates between the MIS and laparotomy arms. Furthermore, the MIS arm showed encouraging perioperative benefits, such as shorter median postoperative hospital stays, lower estimated blood loss, and fewer postoperative complications (2).
In conclusion, minimally invasive surgery will likely gain an incremental role in the management of ovarian cancer. Countless patients with early-stage disease, as well as those with oligometastatic recurrences that have been managed with MIS, have benefited from the substantial perioperative benefits and comparable oncological outcomes. While the role of MIS in advanced ovarian cancer is not yet universally accepted and remains a subject of ongoing discussion due to the current reliance on retrospective data, the preliminary findings from the LANCE trial are highly encouraging. We eagerly await the results from the complete trial, which are expected to provide definitive evidence regarding the long-term oncological efficacy of MIS in this setting.
References
- Jorgensen K, Melamed A, Wu CF, et al. Minimally invasive interval debulking surgery for advanced ovarian cancer after neoadjuvant chemotherapy. Gynecol Oncol. 2023;172:130-137. doi:10.1016/j.ygyno.2023.01.017
- Rauh-Hain JA, Melamed A, Pareja R, et al. Laparoscopic Cytoreduction After Neoadjuvant Chemotherapy in High-Grade Epithelial Ovarian Cancer: A LANCE Randomized Clinical Trial. JAMA Netw Open. 2024;7(11):e2446325. Published 2024 Nov 4. doi:10.1001/jamanetworkopen.2024.46325
About the Author:
Dario R. Roque, MD

Dr. Roque is Chair of the AAGL Oncology SIG, Deputy Editor on the JMIG Editorial Board, and Associate Professor of Gynecologic Oncology in the Department of Obstetrics & Gynecology at Northwestern University Feinberg School of Medicine in Chicago, Illinois.